EU signs joint procurement deal for Covid vaccine

The participating countries will be able to order up to 4 million doses of the protein-based COVID-19 vaccine BIMERVAX, adapted to the LP.8.1 variant, as needed depending on national context and with no minimum number of doses to be bought.
THE CONTRACT WILL RUN FOR A PERIOD OF UP TO TWO YEARS, WITH DELIVERIES OF THE VACCINES IN TIME FOR THE CURRENT VACCINATION SEASON.
"With a sharp rise in COVID-19 cases of the so-called “Frankenstein” variant, we need to ensure that continued protection against this disease is assured, especially to protect the most vulnerable," said Hadja Lahbib, Commissioner for Equality, Preparedness and Crisis Management.
"With a diversified portfolio of vaccines, now including access to up to 4 million doses of this protein-based vaccine, we are enhancing our preparedness and securing a supply of such necessary medical countermeasures against the ever-present threat of COVID-19," she added.
The Commissioner noted that this vaccine from HIPRA follows an end-to-end approach, from R&D to production, located entirely in Europe, strengthening the bloc's strategic autonomy.
While mRNA vaccines are already available, this joint procurement contract diversifies the portfolio to citizens by offering protein-based vaccines.
HIPRA’s vaccine, which has recently received Marketing Authorization from the European Commission after demonstrating that it generates immunity against the LP.8.1 variant and cross-protection against other emerging sublineages such as NB.1.8.1 and XFG, is ready for distribution in single-dose vials and can be stored between 2 °C and 8 °C with a 12-month shelf life. Studies with original vaccine and its adaptation have shown that the vaccine is safe, less reactogenic than mRNA-based vaccine comparator and generates a strong and long-lasting immune response with broad cross-reactivity against emerging variants.
To date, 38 countries have joined the EU's voluntary joint procurement mechanism, which helps member states prepare for health crises and organise joint protection.
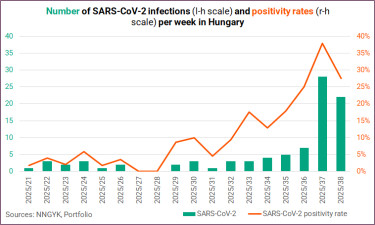
Coronavirus vaccine procurement in Hungary
On Monday, Ágnes Galgóczi, director of prevention and epidemiology at the National Public Health and Medical Centre (NNGYK), told InfoRadio that the “coronavirus vaccine will likely be available in Hungary at the end of October or in November, alongside the flu vaccine”. The article in question also quotes her as saying that "the flu vaccine may arrive at the end of October and the Covid vaccine in November". According to public procurement data, the latter — specifically the end of November — seems to be a realistic arrival date.
The procurement of Covid vaccines was most controversial in Hungary in the past few years.
According to the public procurement database, the NNGYK announced the public procurement for the purchase of new Covid vaccines on September 26. The authority issued the tender in two parts:
- the first part is for vaccines that can be given to people over 12 years of age, of which we would purchase 50,000 doses, plus an optional 10%;
- the second part is for vaccines that can be given to children between 6 months and 12 years of age, of which 800 doses are included in the tender.
The two types of vaccine differ only in the quantity of the otherwise identical composition. A total of 50,800 doses can be delivered, with the option of calling up a further 5,000 doses in a maximum of three instalments by 28 February at the latest.
This is only half of the 100,800 doses purchased last year that included no option for additional doses.
Based on the conditions, it can already be concluded that Moderna and Pfizer will be able to submit valid bids this year.
It would be surprising to see Pfizer return, given that they provided the backbone of the domestic vaccination campaign in previous years before suing the government in January 2023 for refusing to pay for the 3 million specified vaccine doses. The government then switched to Moderna at the end of 2023. However, Pfizer's reappearance is a possibility, as the government had previously rejected Moderna (and then bought vaccines from them at a higher unit price than they were willing to pay Pfizer).
The deadline for submitting bids for this public procurement contract is 28 October. Provided that valid bids are received by this date, the contract can be concluded and delivery can begin once the bids have been evaluated. According to the tender, the storage deadline is 15 calendar days after the contract is signed.
Based on the previous two years, it was possible to get vaccinated around two weeks after the bid submission deadline, so
the second half of November seems realistic unless more than one bid is received, which would delay the decision-making process.
Last year, the NNGYK promised the vaccines by the end of November, but they finally became available in early December — despite the fact that the deadline for submitting bids was later than usual.
In any case, Hungarians - even those in high-risk groups such as elderly people - are not exactly fans of vaccines.
The respiratory season 'officially' starts on the 40th week of the year and 'ends' on the 20th week of the following year. Covid generally dominates in terms of acute respiratory infections at the start of the season, and then the influenza virus takes over.
Covid vaccine, anyone? Elderly Hungarians: Nah, we're good!
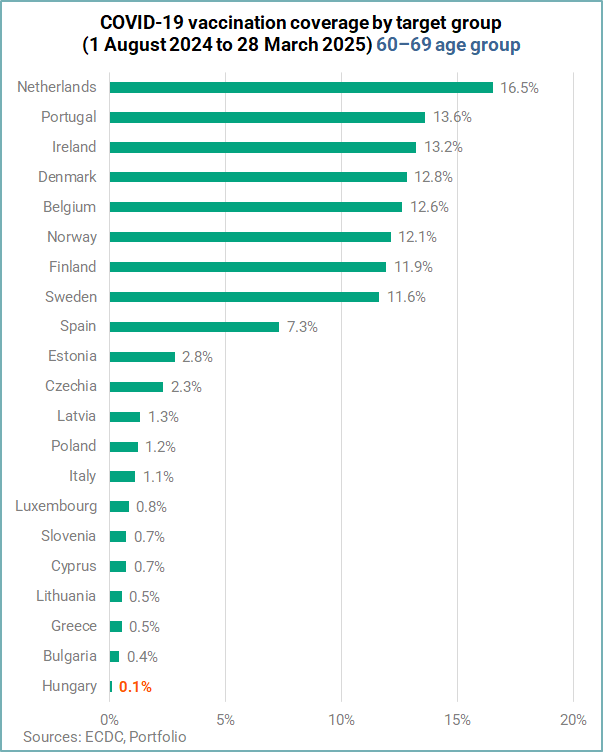
For the age group 60 years and above, approximately 13.6 million people received a dose of COVID-19 vaccine. The median coverage among people in this age group was 8.7%, with high variation across countries. Among the 21 countries reporting data for this target group, none reported a coverage ≥80%.
In the 60-69 age group Hungary had the lowest vaccination coverage rate (VCR) of just 0.1%.
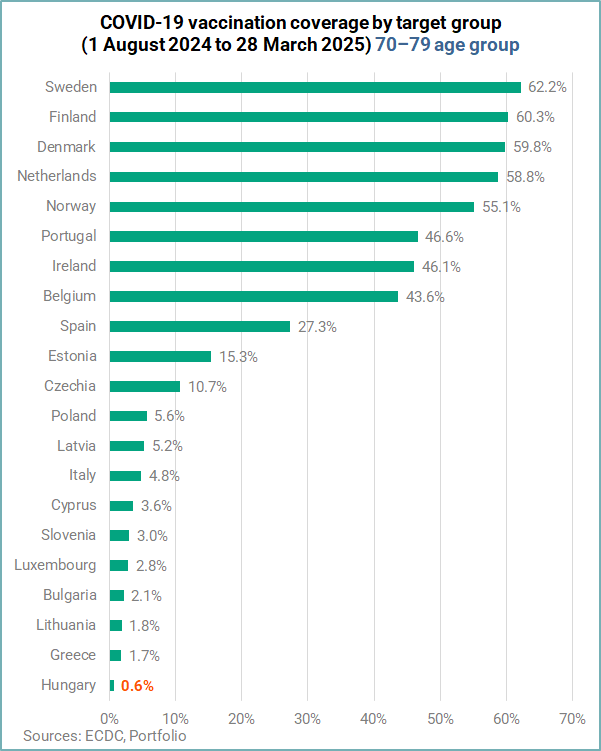
In the 70-79 age group, Sweden (62.2%) and Finland (60.3%) crossed the 60% mark, while
Hungary finished last with an 0.6% VCR.
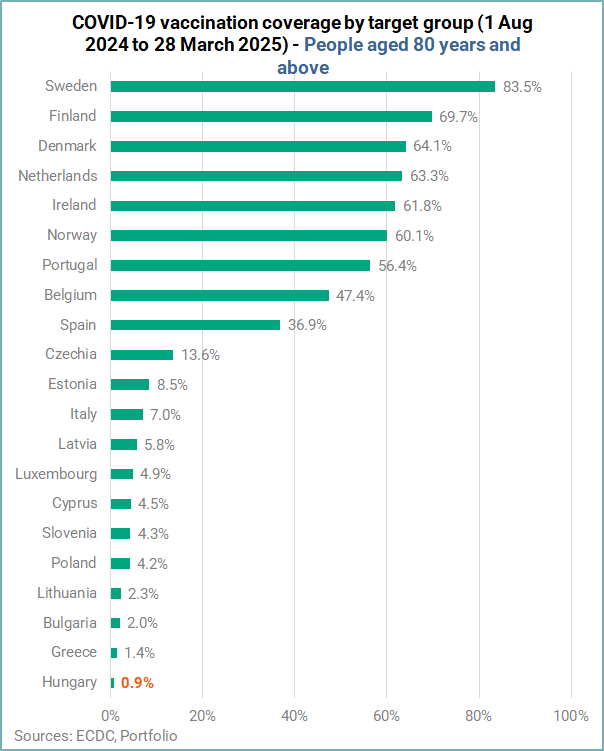
For the age group 80 years and above, approximately four million people received a dose of COVID-19 vaccine. The median coverage among people in this age group was 8.5%, with high variation across countries. Among the 21 countries reporting data for this target group, only one country reported a coverage ≥ 80% (Sweden 83.5%).
Coverage in Hungary was the lowest at just 0.9%.
Cover image (for illustration purposes only): Getty Images